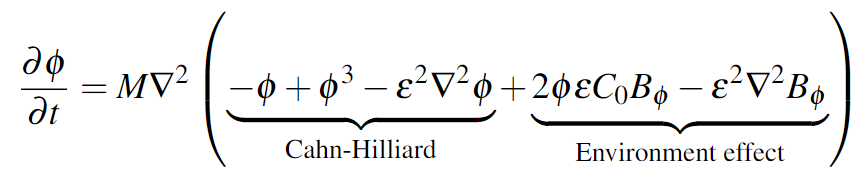Mathematical modelisation of chemotactic cell migration through an extracellular matrix
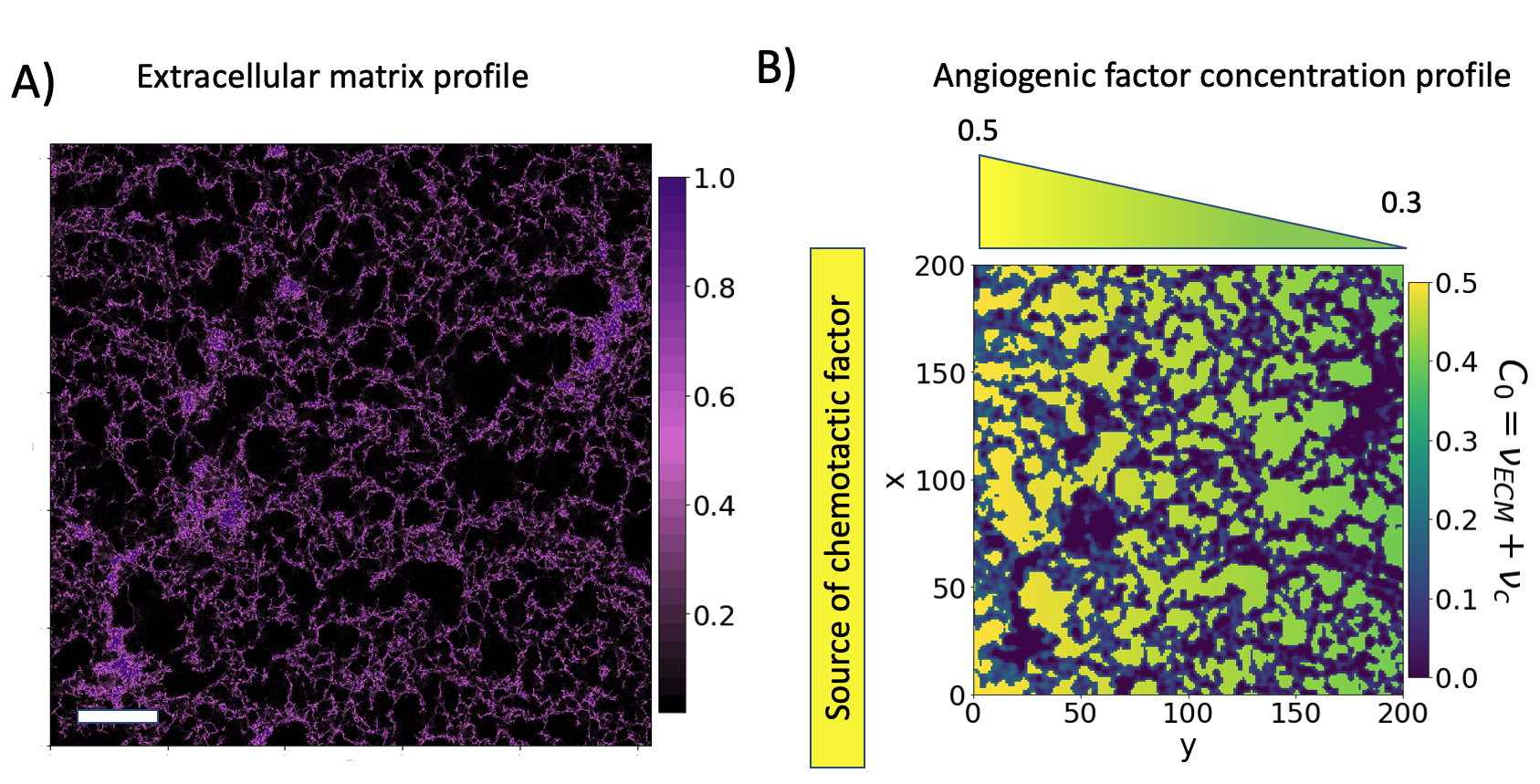
Cell migration plays a fundamental role in veins and arteries development, i.e. vascular development and angiogenesis. Despite cell movement being first seen more than 300 years ago, the mechanisms and identification of the influencing factors behind cell movement are an experimental challenge. In this project, we combined a microfluidic platform that reproduces chemotactic cell migration through the extracellular matrix and a biophysical modelisation through phase-field modelling.
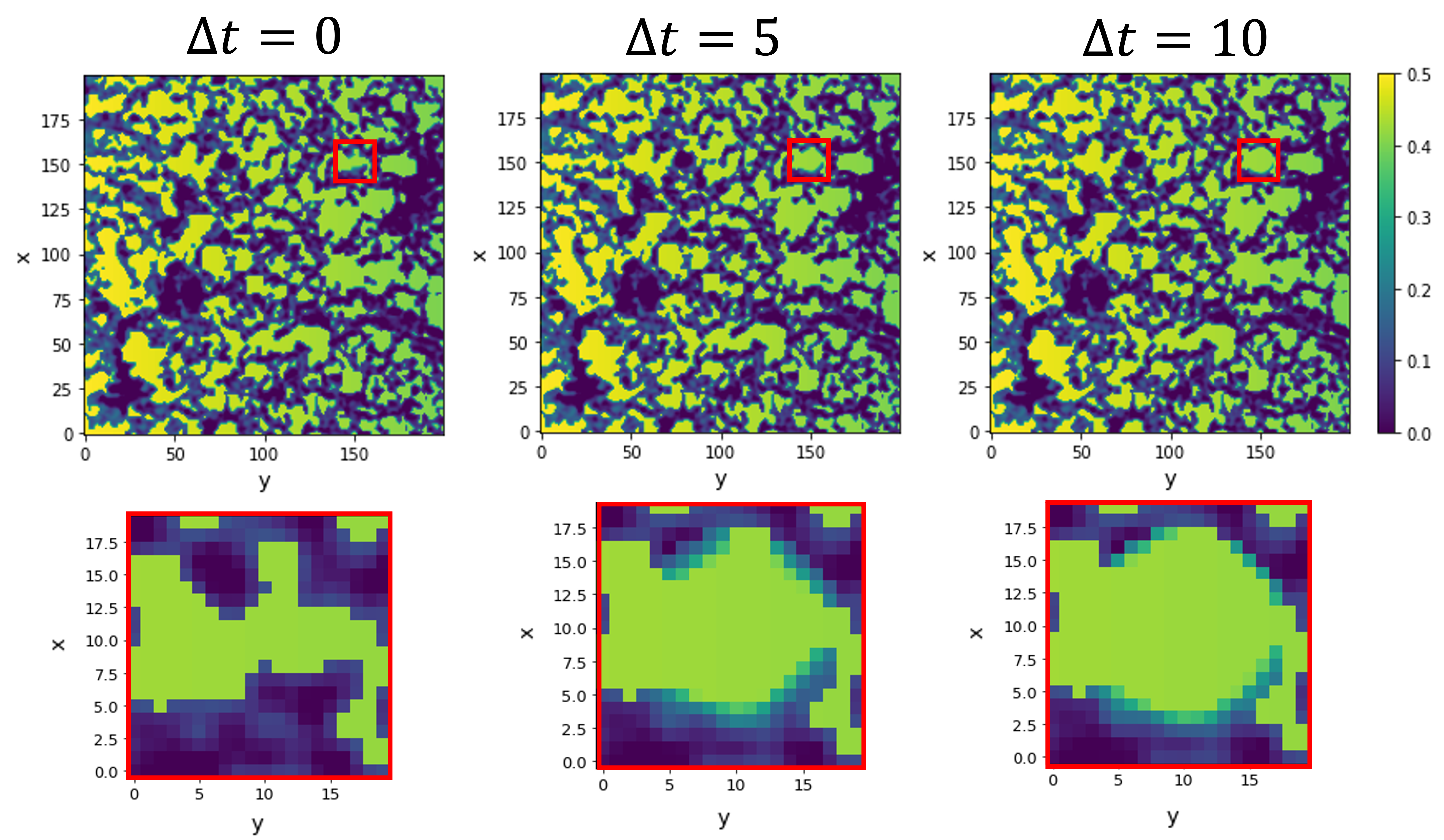
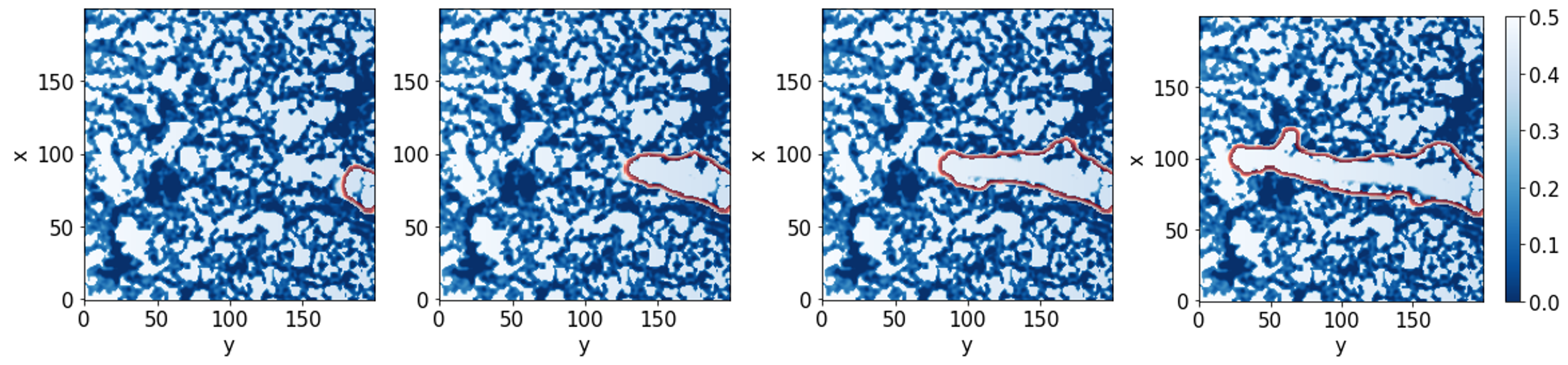
We match theory and experiment by scrutinising the influence of the coupling between the cells and the extracellular matrix. Our model simulates cell migration taking into account the extracellular matrix structure and the distribution of the biological factors.
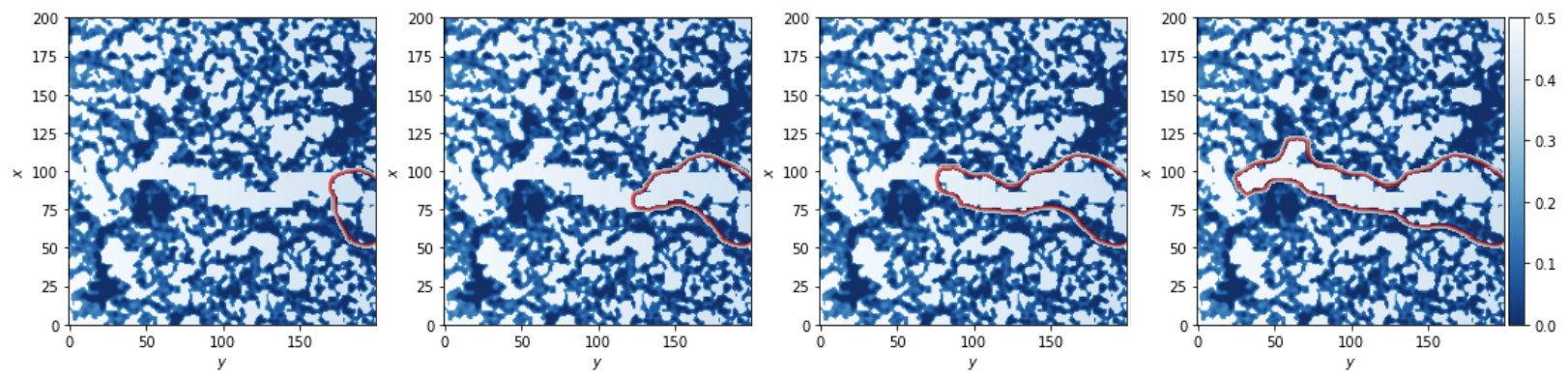
Our findings highlight the inherent nontrivial role of the extracellular structure in the sprouting-invasion dynamics and morphologies.
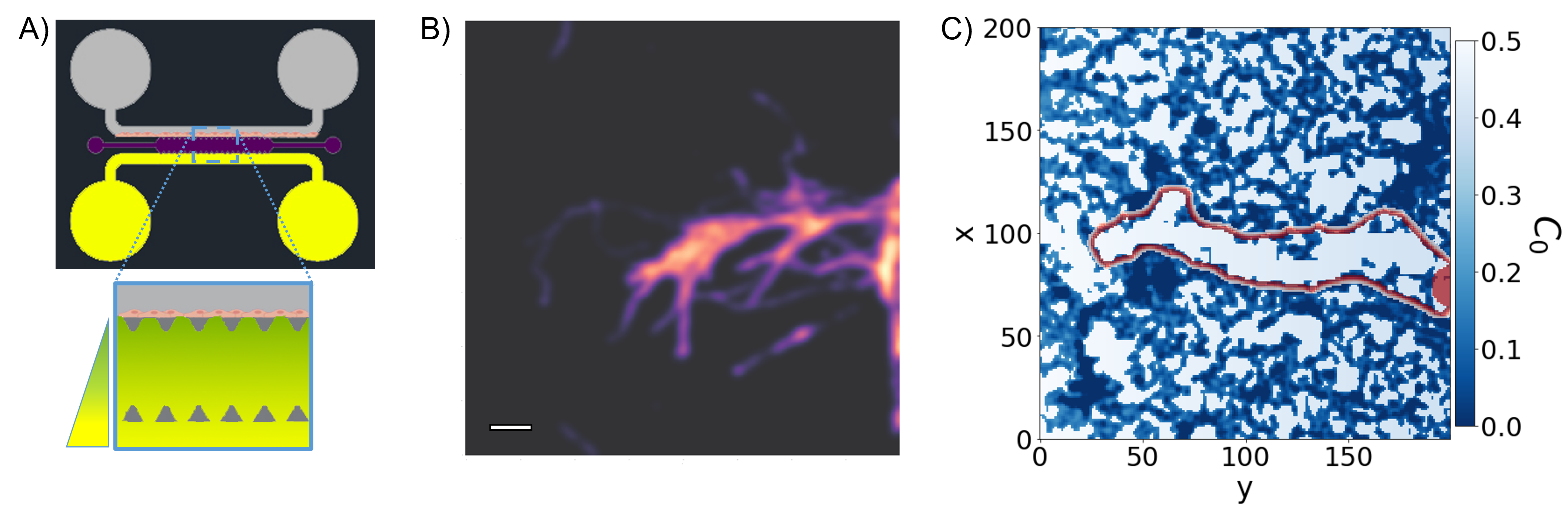
Panel A): experimental setup design. Panel B): cell advancement after 48h (the scale bar is 50μm). Panel C) is a sample of the simulation results.